Manufacturing and Sales of
Medical Devices
In the manufacturing of whiteners and medical devices such as irradiators, we ensure high quality in line with the way in which a medical device manufacturer is expected to manage its product quality. This applies to the entire process from acceptance inspections and shipping inspections to handling post-marketing inquiries from customers.
Planning/Manufacturing

Our expert staff are involved in the development of our original products
We develop whiteners, toothpaste, dental floss and other high value-added products for preventive dentistry. Many of our staff members who are licensed dentists and/or have expertise in a variety of fields are involved in the development of these products.
Promoting innovative development that can lead to
acquisition of regulatory permission and intellectual
property rights (patents)
To ensure that products with advanced technologies, safety and unprecedented added value are released to the world, our medical products and devices such as whiteners and dedicated irradiators acquire regulatory approval and patents.
Committed to safety and whitening effects, we spent nearly eight years developing original whiteners containing hydrogen carbonate and acquired regulatory approval for the use of the product as a specially controlled medical device in December 2018.

Spent eight years acquiring a patent and approval for a product's use as a specially controlled medical device
- Product name
- White Essence Whitening Pro
- Generic name
- Whiteners for dentistry, Class Ⅲ specially controlled medical device
- Medical device approval number
- 23000BZX00368000

Class Ⅱ certification
- Product name
- WE Light Class II
- Generic name
- Whiteners for dentistry, Class III specially controlled medical device
- Medical device approval number
- 228ADBZX00020000
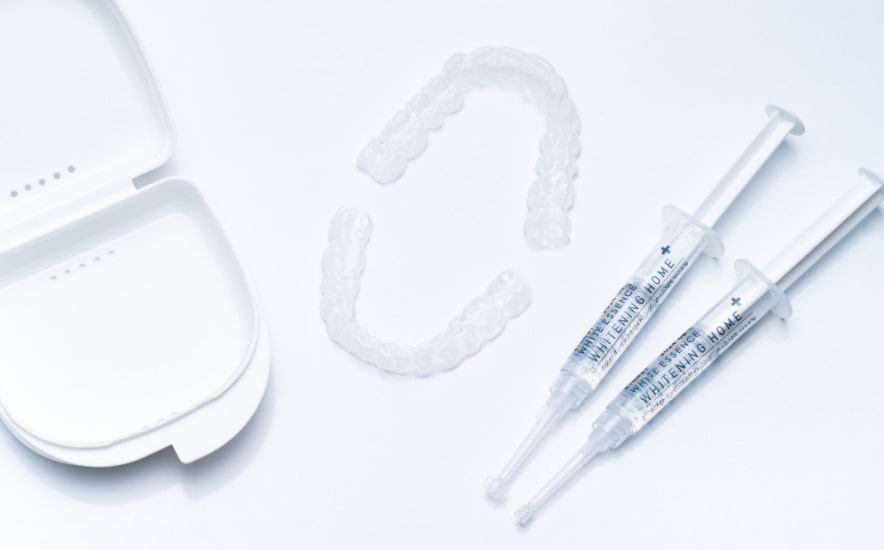
Domestically developed and manufactured to suit the tooth structure of Japanese people
- Product name
- White Essence Whitening Home 10%
- Generic name
- Auxiliary tooth surface cleaner for dental use containing pharmaceutical products, Class III specially controlled medical device
- Medical device approval number
- 30300BZX00311000

Industry-academia cooperation in basic research and applications
We are advancing partnerships with multiple universities in research and development and engage in many different forms of basic research in dental whitening and other practices. We have achieved things in many different areas such as analysis, evaluation and trials conducted in cooperation with universities and are handling inspections by certification agencies and prefectural authorities.
Examples of research content
- Tokyo Medical and Dental University
- Cooperation in basic research and applications for the development of dental whitening
- University of Tsukuba
- Basic research aimed for commercialization of oral biofilm-related products
- Tokyo University of Technology
- Basic research for the elucidation of the mechanism for dental whitening

Developing ultrafine-bubble (UFB) devices using nanosized bubbles that are too small to see
Ultrafine bubbles are nanosized bubbles, 0.000001 millimeter in diameter, which are too small to see. These bubbles enter all corners of dirt to increase the cleaning effect and adsorb to and remove the dirt. We developed in-house an advanced system of ultrafine bubbles which help to remove dirt accumulating in a dental clinic, a space that always consumes water.
Sales and distribution management
Well-received by general dental clinics as well as WHITEESSENCE-affiliated clinics
The high value-added whiteners and medical devices are produced in an innovative development environment. Their sales outlets have been expanded to include general dental clinics in Japan as well as WHITEESSENCE-affiliated clinics and the products are highly regarded. They support the whitening procedures in which the dental hygienist plays a leading role. These products also help dental clinics to improve their operations.
